Cepheid Receives Health Canada Licence for Xpert® Xpress CoV-2 plus
Cepheid Receives Health Canada Licence for Xpert® Xpress CoV-2 plus
Canada NewsWire
SUNNYVALE, Calif., March 15, 2023
"Plus" version of test adds a 3rd gene target to enhance detection of future SARS-CoV-2 variants
SUNNYVALE, Calif., March 15, 2023 /CNW/ -- Cepheid today announced that Health Canada has issued Cepheid a medical device licence for Xpert® Xpress CoV-2 plus, a rapid molecular diagnostic test for qualitative detection of the virus that causes COVID-19.
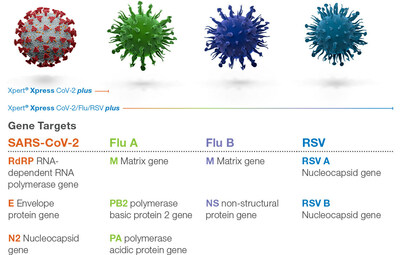
Viruses constantly change through mutation and these mutations can give rise to new variants with unique characteristics. Multiple variants of the virus that causes COVID-19 have been documented globally. Cepheid is proactively addressing this increasing genetic diversity by enhancing gene coverage. The new plus version of the test incorporates a 3rd conserved genetic target for SARS-CoV-2 detection to meet the challenge of future viral mutations and optimizes nucleocapsid gene probes to enable consistent virus detection.
Xpert Xpress CoV-2 plus joins Xpert® Xpress CoV-2/Flu/RSV plus and others in Cepheid's growing portfolio of PCRplus respiratory tests that deliver rapid, accurate, and actionable respiratory results. Xpert Xpress CoV-2/Flu/RSV plus continues to be the most appropriate product for when multiple viruses that cause influenza-like illnesses are circulating. Xpert Xpress CoV2 plus is authorized to be used on any individuals, including for screening those without symptoms or reasons to suspect COVID-19.1
Xpert Xpress CoV-2 plus is designed for use on any of Cepheid's almost 50,000 GeneXpert® systems placed worldwide. The test can provide rapid on-demand detection of SARS-CoV-2 in as soon as 20 minutes for positive results.2
"From the beginning of the pandemic, we have been keenly focused on staying ahead of SARS-CoV-2 genetic drift and have designed our tests in anticipation of current and potential future variants." said David Persing, M.D., Ph.D., EVP, and Chief Scientific Officer. "The high sensitivity of this test is now especially important for recently announced Test-to-Treat initiatives, for which early detection is important for achieving the best clinical outcomes of antiviral therapies."
Xpert Xpress CoV-2 plus is expected to begin shipping to customers in Canada this month.
Visit https://www.cepheid.com/en_CA for more information, videos and package inserts.
*IVD. In Vitro Diagnostic Medical Device. May not be available in all countries.
- PPA and NPA for asymptomatic specimens were calculated using anterior nasal swab specimens
- With early assay termination for positives only; reporting of negatives in approximately 30 minutes
About Cepheid
Based in Sunnyvale, Calif., Cepheid is a leading molecular diagnostics company. Cepheid is dedicated to improving healthcare by developing, manufacturing, and marketing accurate yet easy-to-use molecular systems and tests. By automating highly complex and time-consuming manual procedures, the company's solutions deliver a better way for institutions of any size to perform sophisticated molecular diagnostic testing for organisms and genetic-based diseases. Through its strong molecular biology capabilities, the company is focusing on those applications where accurate, rapid, and actionable test results are needed most, such as managing infectious diseases and cancer. For more information, visit http://www.cepheid.com.
For Cepheid Media Inquiries:
Darwa Peterson
[email protected]
SOURCE Cepheid

![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/March2023/15/c6697.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/March2023/15/c6697.html