AbbVie Receives Positive Recommendation from the Institut national d'excellence en santé et en services sociaux (INESSS) for the Combination VENCLEXTA® With Rituximab as a Treatment for Patients With
AbbVie Receives Positive Recommendation from the Institut national d'excellence en santé et en services sociaux (INESSS) for the Combination VENCLEXTA® With Rituximab as a Treatment for Patients With Chronic Lymphocytic Leukemia
Canada NewsWire
MONTREAL, June 25, 2019
- INESSS recommends the reimbursement of VENCLEXTA® (venetoclax) in combination with rituximab for the treatment of adult patients with chronic lymphocytic leukemia (CLL).i
- Adult patients with CLL taking VENCLEXTA in combination with rituximab can stop their therapy after a defined treatment period of 24 months on treatment.
MONTREAL, June 25, 2019 /CNW/ - AbbVie (NYSE: ABBV), a global research and development-based biopharmaceutical company, today announced that the Institut national d'excellence en santé et en services sociaux (INESSS) recommends that the Minister include Venclexta, in combination with rituximab, on the Lists of Medications for the treatment of chronic lymphocytic leukemia (CLL), if the following conditions are met: exceptional medication and lessening of the economic burden.i VENCLEXTA in combination with rituximab is an effective treatment option that has the benefit of a finite treatment approach, meaning patients are able to stop their therapy after two years of treatment.
"Being able to prescribe a therapy, such as venetoclax in combination with rituximab, that has a finite treatment duration and manageable side effects is a welcomed option for my patients. After two years, I can tell them that they can stop their medication because this effective combination helps to delay disease progression. So far, my clinical experience with venetoclax plus rituximab has been remarkable," explains Dr. Sarit Assouline, Physician, Division of Hematology, Sir Mortimer B. Davis-Jewish General Hospital, Senior Investigator, Lady Davis Institute for Medical Research, Associate Professor, Department of Oncology, McGill University.
INESSS concludes that the evaluation of venetoclax's efficacy and safety in combination with rituximab is based on a good-quality study. The outcomes of that study demonstrated that venetoclax delays disease progression compared to intravenous chemotherapy. Although intravenous chemotherapy is not included on the Lists of Medications, INESSS has recognized its efficacy.i The most common adverse reactions with venetoclax in combination with rituximab were related to white blood cells. Furthermore, there is an unmet clinical need in CLL. The reimbursement of venetoclax in combination with rituximab would be beneficial in the care pathway of CLL patients. i
"The INESSS recommendation for VENCLEXTA plus rituximab is positive news for people living with CLL in Quebec," says Elizabeth Lye, Director of Research & Programs, Lymphoma Canada. "Receiving a diagnosis of CLL or any cancer is always shocking and overwhelming, therefore knowing that there are highly effective treatments available provides reassurance to people facing this uncertain journey."
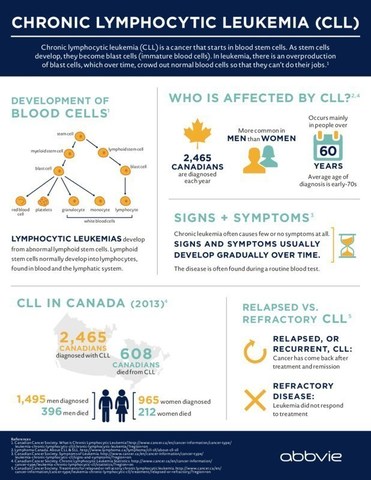
CLL, which is typically a slow-progressing cancer of the bone marrow and bloodii, is one of the most common types of leukemia in adults. In Canada, CLL accounts for approximately 2,465 newly diagnosed cases of leukemia each year and is responsible for more than 600 deaths a year.iii The goal of treatment is to delay progression of the disease and improve quality of life.
"This is another tremendous milestone in our efforts to bring VENCLEXTA plus rituximab to people living with CLL in Quebec. This is a much needed treatment option as it is the first chemotherapy-free combination in CLL that allows patients a 24-month treatment duration," says Stéphane Lassignardie, General Manager of Abbvie Canada.
VENCLEXTA continues to be investigated in CLL and other hematological diseases.
VENCLEXTA is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the U.S. and by AbbVie outside of the U.S.
About the MURANO Study
A total of 389 patients with R/R CLL who had received at least one prior therapy were enrolled in the international, multicenter, open-label, randomized (1:1) MURANO study (NCT02005471). The study was designed to evaluate the efficacy and safety of VENCLEXTA in combination with rituximab (194 patients) compared with bendamustine in combination with rituximab (195 patients). The median age of patients in the trial was 65 years (range 22-85).iv
About AbbVie Care
Canadians prescribed VENCLEXTA will have the opportunity to be enrolled in AbbVie Care, AbbVie's signature care program. The program is designed to provide a wide range of customized services including reimbursement and financial support, pharmacy services, lab work reminders and coordination, personalized education and ongoing disease management support throughout the treatment. For more information, please visit www.abbviecare.ca.
About AbbVie
AbbVie is a global, research and development-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world's most complex and critical conditions. The company's mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. For more information about AbbVie, please visit us at www.abbvie.ca and www.abbvie.com. Follow @abbvieCanada and @abbvie on Twitter or view careers on our Facebook or LinkedIn page.
___________________________________________
i Institut national d'excellence en santé et en services sociaux (INESSS). Avis à la Ministre. www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Mai_2019/Venclexta_2019_05.pdf. Accessed June 2019.
ii Lymphoma Canada. Chronic lymphocytic leukemia. Available at www.lymphoma.ca/lymphoma/lymphoma-101/types-lymphoma/cll. Accessed June 2019.
iii Canadian Cancer Statistics. Chronic lymphocytic leukemia statistics. www.cancer.ca/en/cancer-information/cancer-type/leukemia-chronic-lymphocytic-cll/statistics/?region=on. Accessed June 2019.
iv VENCLEXTA product monograph, AbbVie Corporation. Date of Preparation: September 27, 2016. Date of Revision: February 12, 2019. www.abbvie.ca/content/dam/abbviecorp/ca/en/docs/VENCLEXTA_PM_EN.pdf. Accessed June 2019.

SOURCE AbbVie

View original content to download multimedia: http://www.newswire.ca/en/releases/archive/June2019/25/c7657.html
